

#Atomic mass of boron free
The boron element, which is never found free in nature, forms compounds with different properties with various metals or non-metal elements. Introduction The name Boron comes from the Arabic and Persian words for borax, its principal ore. It is classified as a metalloid due it its properties that reflect a combination of both metals and nonmetals. The rate of the B10 isotope to be found in the nature is 19,1-20,3%, and 79%, 80,9% for B11. Boron is the fifth element of the periodic table (Z5), located in Group 13.
#Atomic mass of boron download
Download 39 Royalty Free Atomic Mass of Boron Vector. Boron consists of two stable isotopes, called B10 and B11 in nature. The best selection of Royalty Free Atomic Mass of Boron Vector Art, Graphics and Stock Illustrations. The boron element, which has semi-metallic and semi-conductive properties, is contained in group 3A of the periodic table.
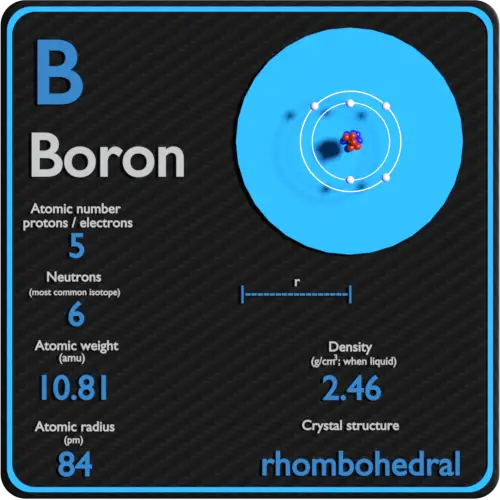
The table in Chapter 21 "Appendix: Periodic Table of the Elements" also lists the atomic masses of the elements.The boron is indicated in the periodic table with B symbol and its atom number is 5 and the atomic weight is 10,81. Carbon exists on Earth as about 99% 12C and about 1% 13C, so the weighted average mass of carbon atoms is 12.01 u. Similar average atomic masses can be calculated for other elements. (also commonly referred to as the atomic weight) of an element.įor example, boron exists as a mixture that is 19.9% 10B and 80.1% 11B. How, then, do we describe the mass of a given element? By calculating an average of an element’s atomic masses, weighted by the natural abundance of each isotope, we obtain a weighted average mass called the atomic mass A weighted average of the masses of all the element’s naturally occurring isotopes. Because most elements exist in nature as a mixture of isotopes, any sample of an element will actually be a mixture of atoms having slightly different masses (because neutrons have a significant effect on an atom’s mass). 'Weighted average' is also called 'weighted mean'. (Im using the version of the definition which I find easier) Relative atomic mass is given the symbol Ar. Note, however, that these masses are for particular isotopes of each element. The relative atomic mass is the weighted average of the masses of the isotopes on a scale on which the mass of a carbon-12 atom is exactly 12 units. For example, the mass of an atom of 1H is 1.008 u, the mass of an atom of 16O is 15.995 u, and the mass of an atom of 32S is 31.97 u. Masses of other atoms are expressed with respect to the atomic mass unit. This is the reason why the mass of a boron atom is 10.81 u despite not having a boron atom with a mass of 10.81 u.
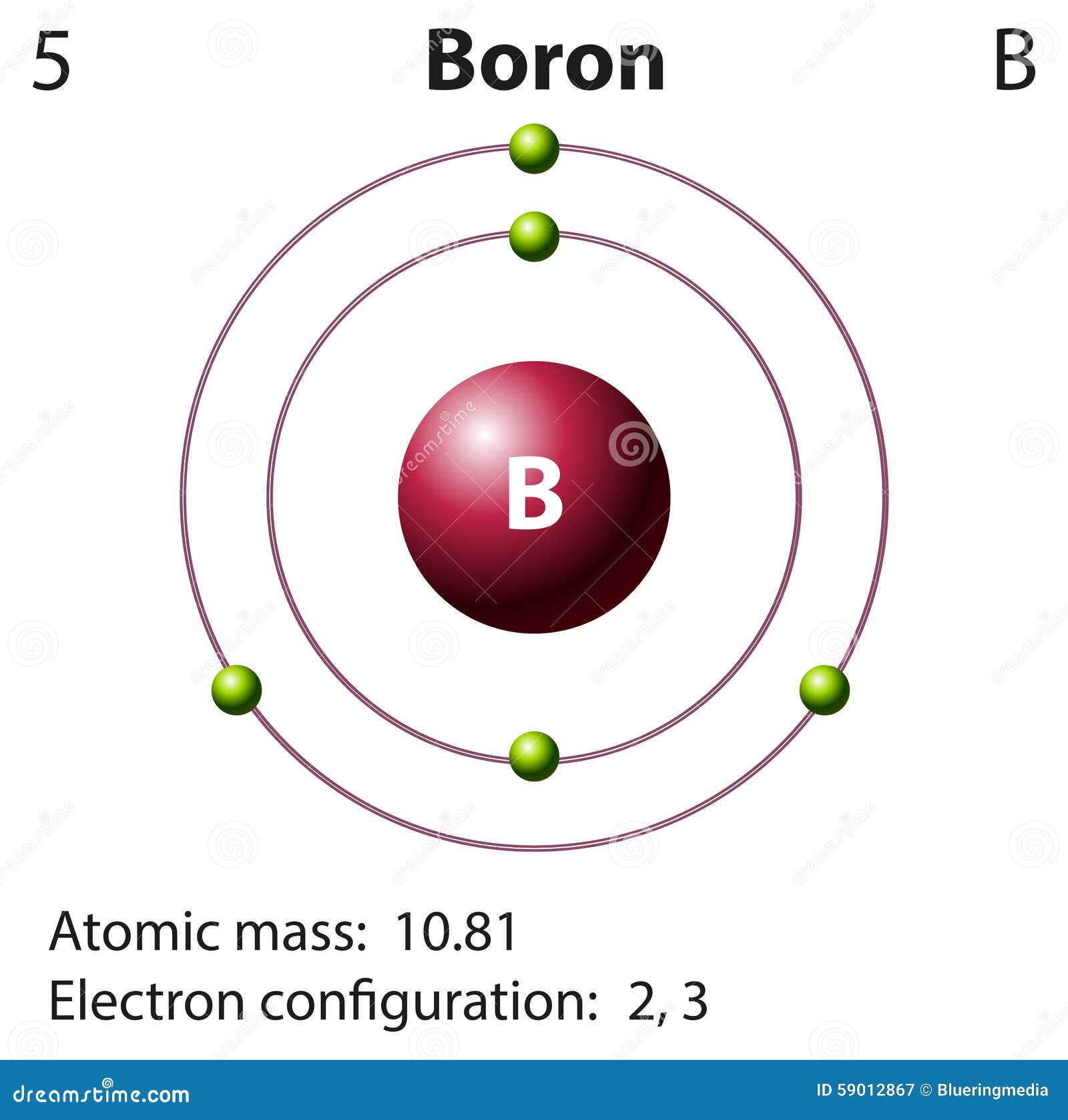
(abbreviated u, although amu is also used) is defined as 1/12 of the mass of a 12C atom: 1 u = 1 12 the mass of 12 C atom The atomic mass unit One-twelfth the mass of a 12C atom. Their masses are so small, however, that chemists often use a unit other than grams to express them-the atomic mass unit. Define atomic mass and atomic mass unit.Įven though atoms are very tiny pieces of matter, they have mass.


 0 kommentar(er)
0 kommentar(er)
